Clemmensen reduction:
The Clemmensen reduction provides an identical product but is performed under acid conditions and thus this is a appropriate method for compounds that are not stable to basic conditions.
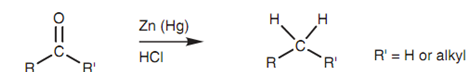
Figure: Clemmensen reduction.
Compounds that are sensitive to both acid and base can be reduced within neutral conditions by creating the thioacetal or thioketal, after that reducing with Raney nickel as shown in figure.
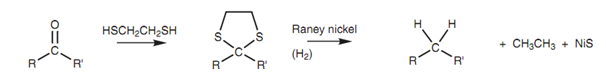
Figure: Reduction via a cyclic thioketal.
Aromatic aldehydes and ketones can as well be deoxygenated along with hydrogen over a palladium charcoal catalyst. The reaction occurs because the aromatic ring activates the carbonyl group towards reduction. Aliphatic aldehydes and ketones are not reduced.
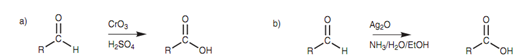
Figure: (a) Oxidation of an aldehyde to form a carboxylic acid; (b) Oxidation of an aldehyde using silver oxide.