Alkenes to Aldehydes and Ketones:
Treating an alkene along with ozone as displayed in figure results in oxidation of the alkene and creation of an initial ozonide which after that again arranges to an isomeric ozonide. This second ozonide is unstable and potentially explosive and thus it is not generally isolated.
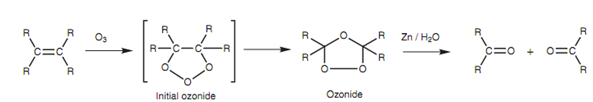
Figure: Ozonolysis of an alkene.
Instead, it is decreased with zinc and water resultant in the formation of two separate molecules. The alkene is split across the double bond to provide two carbonyl compounds. These will be ketones or aldehydes depending upon the substituents present. For instance, 3-methyl-2-pentene provides an aldehyde and a ketone.
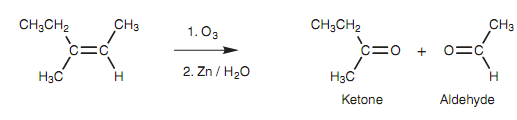
Figure: Ozonolysis of 3-methyl-2-pentene.