Alkenes to1, 2-diols:
The reaction of alkenes along with osmium tetroxide (OsO4) is other example of an oxidation reaction
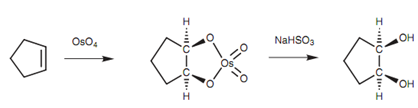
Figure: syn-Hydroxylation of an alkene.
Though, in this case the alkene is not split. Instead, a 1, 2-diol is acquired - also termed as a glycol. The reaction involves the creation of a cyclic intermediate in which the osmium reagent is attached to one face of the alkene. On treatment with sodium bisulfite, the intermediate is cleaved like that the two oxygen atoms connecting the osmium remain attached. This results in both alcohols being added to similar side of the double bond - syn-hydroxylation.
Similar reaction can as well be performed using cold alkaline potassium permanganate (KMnO4) followed via treatment with aqueous base.
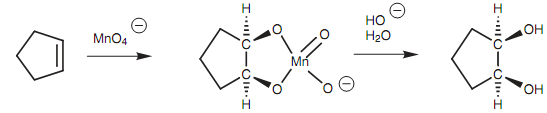
Figure: syn-Hydroxylation with KMnO4.
It is significant to keep the reaction cold because potassium permanganate can cleave the diol through further oxidation. The reaction acts better with osmium tetroxide. Though, this is a highly toxic and expensive reagent and has to be handled with care. Anti-hydroxylation of the double bond can be acquired by creating an epoxide, then performing an acid-catalyzed hydrolysis.