Steric factors:
Steric factors can play a vital part in the reactivity of acid derivatives. For instance, a bulky group linked to the carbonyl group can hinder the approach of nucleophiles and therefore lower reactivity. The steric bulk of the nucleophile can as well have an effect in slowing down the reaction. For instance, acid chlorides react faster with primary alcohols as compared they do with secondary or tertiary alcohols. This permits selective esterification if a molecule has much more than one alcohol group present shown in figure.
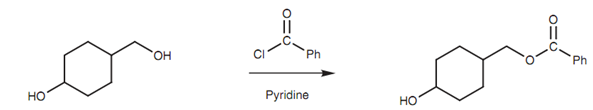
Figure: Selective esterification of a primary alcohol.