Reduction
Carboxylic acids, acid chlorides, acid anhydrides and esters are reduced to primary alcohols on treatment with lithium aluminum hydride (LiAlH4).
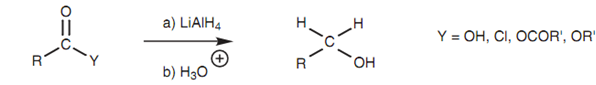
Figure: Reduction of acid chlorides, acid anhydrides, and esters with lithium aluminum hydride.
The reaction includes nucleophilic substitution through a hydride ion to provide an intermediate aldehyde. This cannot be isolated because the aldehyde immediately goes through a nucleophilic addition reaction with other hydride ion.
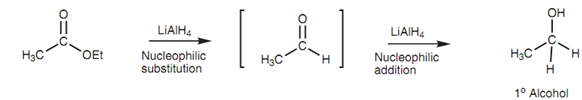
Figure: Intermediate involved in the LiAlH4 reduction of an ester.
The detailed mechanism is as displayed in figure.
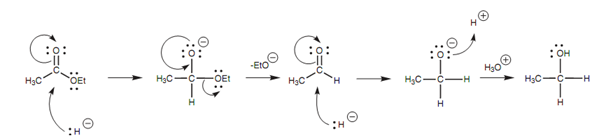
Figure: Mechanism for the LiAlH4 reduction of an ester to a primary alcohol.