Organolithium reactions:
Esters react along with two equivalents of an organolithium reagent to provide a tertiaryalcohol in which two of the alkyl groups are derived from the organolithium reagent as shown in diagram. The mechanism of the reaction is similar as that explained in the Grignard reaction, i.e. nucleophilic substitution to make a ketone followed by nucleophilic addition. It is essential to protect any carboxylic acids present while performing organolithium reactions because one equivalent of the organo- lithium reagent would be wasted in an acid-base reaction along with the carboxylic acid.
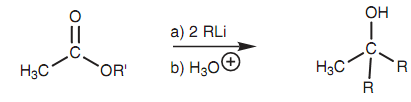
Figure: Reaction of an ester with an organolithium reagent to form a tertiary alcohol.