Organocuprate reactions:
Acid chlorides react along with diorganocuprate reagents to make ketones. As the Grignard reaction, an alkyl group displaces the chloride ion to generate a ketone. Though, not like the Grignard reaction, the reaction ends at the ketone stage. The mechanism is considered to be radical based as compared to a nucleophilic substitution. This reaction does not occur with carboxylic acids, esters, acid anhydrides, or amides.
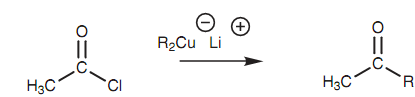
Figure: Reaction of an acid chloride with a diorganocuprate reagent to produce a ketone.