Mechanism of hydrolysis of ethyl ethanoate:
Esters and amides are much less reactive and thus the hydrolysis needs more forcing conditions by using aqueous sodium hydroxide or aqueous acid with heating.
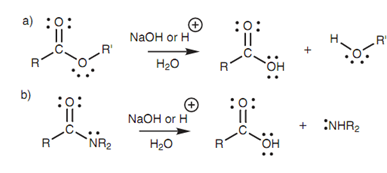
Figure: Hydrolysis of (a) esters; (b) amides.
Under fundamental conditions, the hydroxide ion works as the nucleophile through the normal mechanism for nucleophilic substitution. For instance, the mechanism of hydrolysis of ethyl acetate is as displayed in figure.
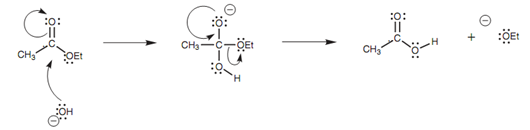
Figure: Mechanism of hydrolysis of ethyl ethanoate.
Though, the mechanism does not end here. The carboxylic acid that is formed reacts with sodium hydroxide to make a water soluble carboxylate ion. Additionally, the ethoxide ion that is lost from the molecule is a stronger base than water and goes through proto-nation. The basic hydrolysis of an ester is as well termed as saponi?cation and generates a water soluble carboxylate ion.
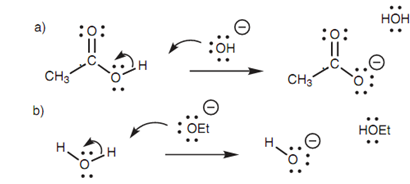
Figure: (a) Ionization of a carboxylic acid; (b) neutralization of the ethoxide ion.
Similar mechanism is involved in the basic hydrolysis of an amide and as well results in a water soluble carboxylate ion. The leaving group from an amide is initially charged (that is R2N:-). Though, this is a strong base and reacts with water to make a free amine plus a hydroxide ion.
In the basic hydrolysis of esters and amides, the creation of a carboxylate ion is irreversible and thus serves to drive the reaction to completion. To isolate the carboxylic acid rather than the salt, it is essential to add acid (for example dilutes HCl) to the aqueous solution. The acid protonates the carboxylate salt to provide the carboxylic acid which (in several cases) is no longer soluble in aqueous solution and precipitates out like a solid or as an oil.