Mechanism for the acid-catalyzed hydrolysis of an ester:
Mechanism for acid-catalyzed hydrolysis shown in diagram involves water acting like a nucleophile. Though, water is a poor nucleophile because it gains an unfavorable positive charge while it forms a bond. Hence, the carbonyl group has to be activated which takes place when the carbonyl oxygen is protonated through the acid catalyst (Step 1). Nucleophilic attack via water is now favored because it neutralizes the unfavorable positive charge on the carbonyl oxygen (Step 2). The intermediate comprises a positive charge on the oxygen derived from water, but this is neutralized through losing the attached proton like that the oxygen gains the electrons in the O-H bond (Step 3). Other protonation now occurs (Step 4). This is essential in order to convert a poor leaving group (the methoxide ion) into a good leaving group (methanol). The π bond can now be again formed (Step 5) with loss of methanol. Lastly, water can act like a base to remove the proton from the carbonyl oxygen (Step 6).
The ester's acid-catalyzed hydrolysis is not as effective as basic hydrolysis because all the steps in the mechanism are reversible and there is no salt formation to pull the reaction through to products. Hence, it is significant to use an excess of water in order to shift the equilibria to the products. In difference to esters, the hydrolysis of an amide in acid does result in the creation of an ion. The leaving group here is an amine and because amines are basic, they will react along with the acid to form a water soluble aminum ion. This is an irreversible step that pulls the equilibrium through to the products.
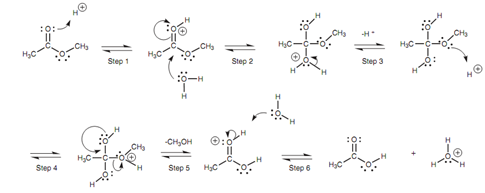
Figure: Mechanism for the acid-catalyzed hydrolysis of an ester.