Grignard reaction
Esters and Acid chlorides react with two equivalents of a Grignard reagent to generate a tertiary alcohol in which two extra alkyl groups are provided through the Grignard reagent as shown in figure.
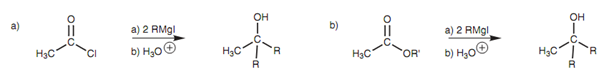
Figure: Grignard reaction with (a) an acid chloride; and (b) an ester to produce a tertiary alcohol.
There are 2 reactions that are included in this process. The acid chloride reacts with the 1st equivalent of Grignard reagent in a general nucleophilic substitution to generate an intermediate ketone. Though, this ketone is as well reactive to Grignard reagents and immediately reacts along with a second equivalent of Grignard reagent by the nucleophilic addition mechanism that explained for aldehydes and ketones.
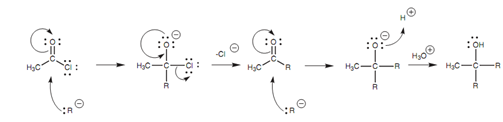
Figure: Mechanism of the Grignard reaction with an acid chloride.
Carboxylic acids react with Grignard reagents in an acid-base reaction resultant in creation of the carboxylate ion and creation of an alkane from the Grignard reagent. This has no synthetic use and it is significant to protect carboxylic acids while performing Grignard reactions on another part of the molecule thus that the Grignard reagent is not wasted.
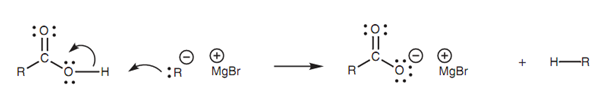
Figure: Acid-base reaction of a Grignard reagent with a carboxylic acid.