Dehydration of primary amides:
Primary amides are dehydrated to nitriles by using a dehydrating agent like thionyl chloride (SOCl2), phosphoryl trichloride (POCl3), phosphorus pentoxide (P2O5), or acetic anhydride shown in figure.
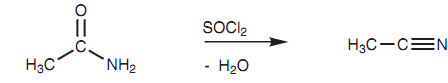
Figure: Conversion of a primary amide to a nitrile.
The mechanism for the dehydration of an amide along with thionyl chloride is displayed in figure. Even though the reaction is the equivalent of dehydration, the mechanism depicts that water itself is not eliminated. The reaction is occurs by the loss of sulfur dioxide like a gas.
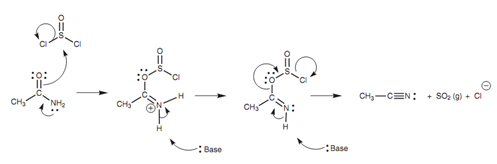
Figure: Mechanism for the dehydration of a primary amide to a nitrile.