Oxidation:
Phenols are susceptible to oxidation to quinones as displayed in figure.
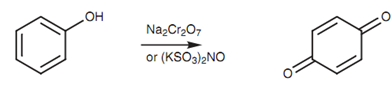
Figure: Oxidation of phenol.
Claisen rearrangement:
A helpful method of introducing an alkyl substituent to the ortho position of a phenol is through the Claisen rearrangement. The phenol is transformed to the phenoxide ion, then treated with 3-bromopropene (an allyl bromide) to make an ether. On heating, the allyl group (-CH2-CH=CH2) is transferred from the phenolic group to the ortho position of the aromatic ring.
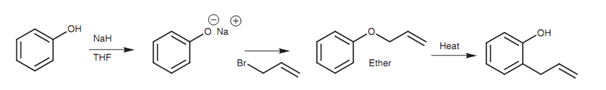
Figure: Claisen rearrangement.
The mechanism includes a concerted process of bond formation and bond breaking termed as a pericyclic reaction. This results in a ketone structure that immediately tautomerizes to the last product.
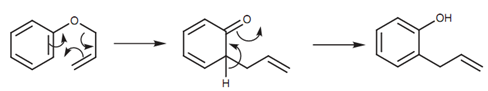
Figure: Mechanism for the Claisen rearrangement.
Dissimilar allylic reagents could be employed in the reaction and the double bond in the ?nal product could be reduced to make an alkane substituent without influencing the aromatic ring.