Functional group transformations:
Phenols can be transformed into esters via reaction with acid chlorides or acid anhydrides, and into ethers by reaction with alkyl halides in the existence of base. These reactions can be performed under milder conditions than those employed for alcohols because of the greater acidity of phenols.
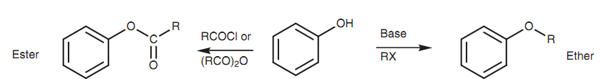
Figure: Functional group transformations for a phenol.
Thus phenols can be transformed to phenoxide ions with sodium hydroxide rather than metallic sodium.
Even though the above reactions are common to alcohols and phenols, there are various reactions that can be performed on alcohols but not phenols, and vice versa. For instance, unlike alcohols, phenols cannot be transformed to esters by reaction with a carboxylic acid within acid catalysis. Reactions including the cleavage of the C-O bond are as well not possible for phenols. The aryl C-O bond is stronger as compared to the alkyl C-O bond of an alcohol.