Electrophilic substitution:
Electrophilic substitution is promoted through the phenol group which acts like an activating group and leads substitution to the ortho and para positions. Sulfonation and nitration of phenols are both probable to provide ortho and para substitution products. On occasions, the phenolic groups might be very much powerful an activating group and it is hard to control the reaction to one substitution. For instance, the bromination of phenol directs to 2, 4, 6-tribromophenol even in the nonexistence of a Lewis acid.
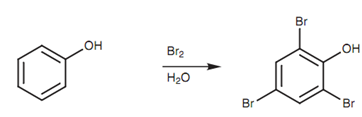
Figure: Bromination of phenol
The activating power of the phenolic group can be get decreased by transforming the phenol to an ester that can be removed by hydrolysis once the electrophilic substitution reaction has been performed. As the ester is a weaker activating group, substitution take place only once. Furthermore, because the ester is a bulkier group than the phenol, para substitution is favored over ortho substitution.
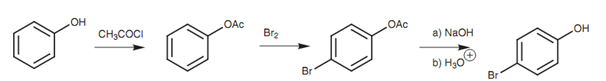
Figure: Synthesis of para-bromophenol.