Thioethers:
Not like ethers, thioethers make good nucleophiles because of the sulfur atom. This is since the sulfur atom has the valence electrons of it further away from the nucleus. The result of it is tha these electrons experience less attraction from the nucleus, creating them more polarizable and more nucleophilic. Because they are good nucleophiles, thioethers can react with alkyl halides to make trialkylsulfonium salts (R3S+) - a reaction that is not possible for normal ethers.
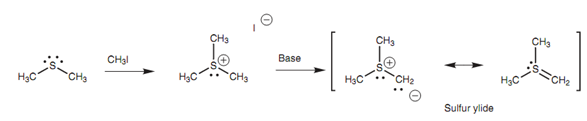
Figure: Formation of a sulphur ylide.
Sulfur is as well capable to stabilize a negative charge on a neighboring carbon atom, particularly while the sulfur itself is positively charged. This forms the protons on neighboring carbons acidic, permitting them to be removed with base to make sulfur ylides.
Sulfur ylides are helpful in the synthesis of epoxides from ketones or aldehydes. They go through a typical nucleophilic addition with the carbonyl group to make the supposed tetrahedral intermediate.
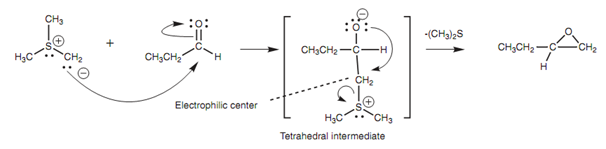
Figure: Reaction of an aldehyde with a sulfur ylide to produce an epoxide.
Now this intermediate has an extremely good thioether leaving group that also creates an electrophilic carbon atom at the neighboring position. Hence, the molecule is set up for further reaction that involves the nucleophilic oxygen anion displacing the thioether and creating an epoxide.