Epoxides:
Epoxides are cyclic ethers, however they are more reactive than normal ethers due to the ring strain involved in a three-membered ring. Hence, ring opening by an SN2 nucleophilic substitution is a typical reaction of epoxides.
For instance, epoxides can be ring-opened under acidic or basic conditions to provide a 1, 2-diol.
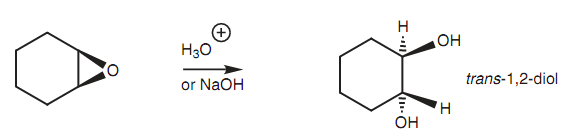
Figure: Ring opening of an epoxide under acidic or basic conditions.
In both of the cases, the reaction includes an SN2 mechanism along with the incoming nucleophile attacking the epoxide from the reverse direction of the epoxide ring. This causes in a trans arrangement of the diol system while the reaction is performed on cycloalkane epoxides. Within acidic conditions as shown in figure, the epoxide oxygen is ?rst protonated, turning it into a better leaving group (Step 1). After that water acts as the nucleophile and attacks one of the electrophilic carbon atoms of the epoxide. Water employs a lone pair of electrons to make a new bond to carbon and as it does so, the C-O bond of the epoxide cleaves along with both electrons moving onto the epoxide oxygen to neutralize the positive charge (Step 2).
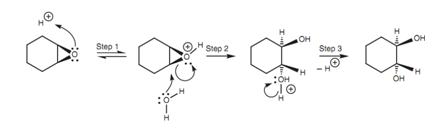
Figure: Mechanism for the ring opening of an epoxide under acidic conditions.
Even though water is a poor nucleophile, the reaction is favored because of the neutralization of the positive charge on oxygen and the relief of ring strain one time the epoxide is opened up. Not like other SN2 reactions of course, the leaving group is still tethered to the molecule.