Cleavage of a tertiary ether:
The initial protonation is necessary as it converts a poor leaving group (an alkoxide ion) into a good leaving group (the alcohol). Primary alcohols made from this reaction may be transformed further to an alkyl halide. Tertiary ethers react through the SN1 mechanism to generate the alcohol. Though, an alkene may also be formed because of E1 elimination and this might be the major product.
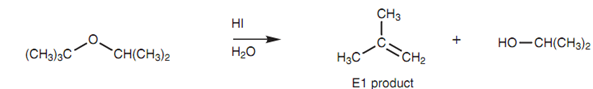
Figure: Cleavage of a tertiary ether.
Tri?uoroacetic acid can be employed instead of HX, resultant in an E1 reaction and production of the alcohol and the alkene.
A difficulty with most ethers is their slow reaction along with atmospheric oxygen through a radical process to make hydroperoxides (ROOH) and peroxides (ROOR). These products can prove to be explosive if old solvents are concentrated to dryness.