Nucleophilic and electrophilic centers:
The complete reaction includes the replacement of an α-proton with an alkyl group. The electrophilic and nucleophilic centers of the enolate ion and methyl iodide are displayed in figure. The enolate ion comprises its negative charge shared among the oxygen atom and the carbon atom because of resonance, and thus both of these atoms are nucleophilic centers. Iodomethane has a polar
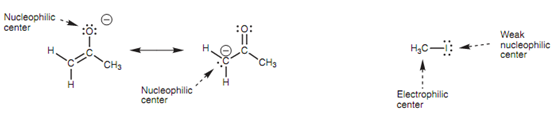
Figure: Nucleophilic and electrophilic centers.
C-I bond in which the iodine is a weak nucleophilic center and the carbon is a very good electrophilic center.
One possible reaction among these molecules includes the nucleophilic oxygen (o2) by using one of its lone pairs of electrons to make a new bond to the electrophilic carbon on iodomethane. At similar time, the C-I bond and both electrons move onto iodine to provide it a fourth lone pair of electrons and a negative charge. This reaction is probable, but in practice the product acquired is more likely to occur from the reaction of the alternative carbanion structure that reacting with methyl iodide. This is a much more helpful reaction since it includes the formation of a carbon-carbon bond and permits the construction of much more complex carbon skeletons.
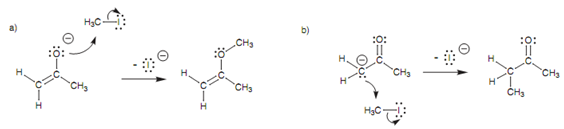
Figure: (a) O-Alkylation; (b) C-alkylation.