Mechanism for C-alkylation:
Alternative mechanism to that displayed in figure, but that gives the same result, begins with the enolate ion. The enolate ion is much more stable as compared to the carbanion because the charge is on the electronegative oxygen and thus it is more likely that the reaction mechanism will take place in this way. This is a extremely useful reaction in organic synthesis. Though, there are limitations to the type of alkyl halide that can be employed in the reaction.
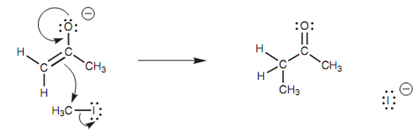
Figure: Mechanism for C-alkylation of the enolate ion.
The reaction is SN2 with respect to the alkyl halide and thus the reaction acts best with primary alkyl, primary benzylic, and primary allylic halides. The enolate ion is a strong base and if it is reacted along with secondary and tertiary halides, elimination of the alkyl halide occurs to provide an alkene.
α-Alkylation acts well with ketones, but not very well for aldehydes because the latter tend to go through Aldol condensations instead. The α-protons of a ketone like propanone are just only weakly acidic and thus a powerful base (for example lithium diisopropylamide) is needed to generate the enolate ion needed for the alkylation. An alternative mechanism of preparing similar product but using a milder base is to begin with ethyl acetoacetate (a β-keto ester) instead.