Decarboxylation mechanism:
In this structure the α-protons are more acidic because they are flanked through two carbonyl groups. The result of it is that the enolate can be made by using a weaker base like sodium ethoxide. One time the enolate has been alkylated, the ester group can be hydrolyzed and decarboxylated on heating along with aqueous hydrochloric acid. The decarboxylation mechanism includes the β-keto group and would not take place if this group was not present. Carbon dioxide is lost and the enol tautomer is created. After that this can form the keto tautomer via the normal keto-enol tautomerism.
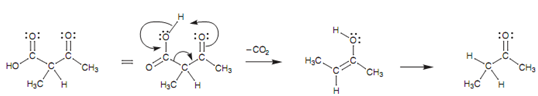
Figure: Decarboxylation mechanism.
It is probable for two dissimilar alkylations to be performed on ethyl acetoacetate because there is much more than one α-proton present. β-keto esters like ethylacetoacetate are as well helpful in solving a problem included in the alkylation of unsymmetrical ketones.
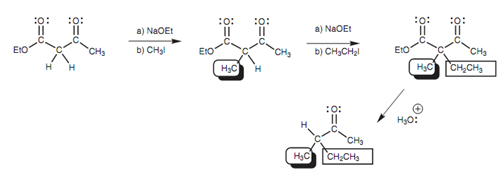
Figure: Double alkylation of ethylacetoacetate.
For instance, alkylating butanone along with methyl iodide leads to two dissimilar products because there are α-protons on either side of the carbonyl group.