Aldol reaction:
Enolate ions can as well react with aldehydes and ketones through nucleophilic addition. The enolate ion works as the nucleophile while the aldehyde or ketone works as an electrophile. Because the enolate ion is created from a carbonyl compound itself, and can after that react with a carbonyl compound, it is feasible for an aldehyde or ketone to react with itself. We can demonstrate this via looking at the reaction of acetaldehyde with sodium hydroxide. Within these conditions, two molecules of acetaldehyde are related together to make a β-hydroxyaldehyde.
Within this reaction, two separate reactions are going on - the creation of an enolate ion from one molecule of acetaldehyde, and addition of that enolate to a 2nd molecule of acetaldehyde.
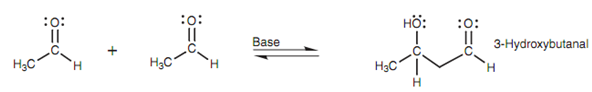
Figure: Aldol reaction.