Elimination:
Primary amines could be transformed to alkenes if it was probable to eliminate ammonia from the molecule. Though, the direct elimination of ammonia is not feasible. A less direct method of acquiring similar result is to exhaustively methylate the amine through the SN2 reaction to provide a quaternary ammonium salt. Once this is made, it is feasible to eliminate triethylamine in the existence of silver oxide and to form the needed alkene. The reaction is termed as the Hofmann elimination.

Figure: Hofmann elimination.
The silver oxide gives a hydroxyl ion which works as the base for E2 elimination that s described in figure.
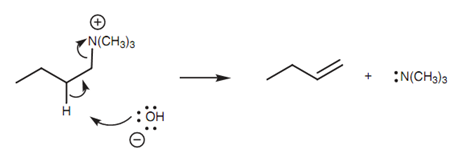
Figure: Mechanism of the Hofmann elimination.
Though, unlike most E2 eliminations, the less substituted alkene is chosen if a choice is available.