Electrophilic aromatic substitution:
Aromatic amines like aniline go through electrophilic substitution reactions in which the amino group works like a strongly activating group, directing substitution to the ortho and para positions. Such as phenols, the amino group is such a strong activating group that more than one substitution may occur. For instance, reaction of aniline with bromine results in a tribrominated structure since the only product. This problem can be conquers by transforming the amine to a less activating group. Generally, this includes acylating the group to generate an amide. This type of group is much weaker activating group and thus mono-substitution occurs. Furthermore, because the amide group is bulkier as compared to the original amino group, there is more of a preference for para substitution on ortho substitution. One time the reaction has been performed, the amide can be hydrolyzed back to the amino group.
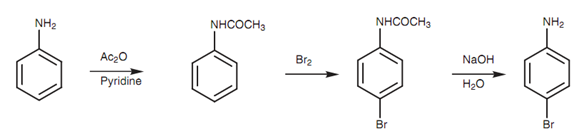
Figure: Synthesis of para-bromoaniline.
Anilines can be nitrated and sulfonated, however the Friedel-Crafts alkylation and acylation are not possible because the amino group forms an acid base complex along with the Lewis acid needed for this reaction. One way round this is to transform the aniline to the amide as above before performing the reaction.