Synthesis of a disubstituted alkyne:
It is as well possible to construct larger carbon skeletons by using alkyl halides. A simple instance is the reaction of an alkyl halide with a cyanide ion shown in figure. This is a significant reaction because the nitrile product can be hydrolyzed to provide a carboxylic acid.
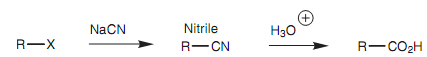
Figure: Constructing larger carbon skeletons from an alkyl halide.
The reaction of an acetylide ion along with a primary alkyl halide permits the synthesis of disubstituted alkynes. The enolate ions of esters or ketones can as well be alkylated with alkyl halides to make larger carbon skeletons. The very much successful nucleophilic substitutions are with primary alkyl halides. The elimination reaction might compete, particularly when the nucleophile is a strong base, with secondary and tertiary alkyl halides. The substitution of tertiary alkyl halides is very well done in a protic solvent with weakly basic nucleophiles. Though, yields may be poor.

Figure: (a) Synthesis of a disubstituted alkyne; (b) alkylation of an enolate ion.