Eliminations:
Alkyl halides' (dehydrohalogenations) elimination reactions are a helpful method of synthesizing alkenes. For best results, a strong base (for example NaOEt) should be employed in a protic solvent (EtOH) with the secondary or tertiary alkyl halide. The reaction carries on through an E2 mechanism. Heating raises the chances of elimination over substitution.
For primary alkyl halides, a strong, bulky base (for example NaOBut) should be employed. The bulk hinders the chance of the SN2 substitution and encourages elimination via the E2 mechanism. The advantage of the E2 mechanism is that it is higher yielding than the E1 mechanism and is as well stereo specific. The geometry of the product acquired is ascertained by the antiperiplanar geometry of the transition state. For instance, the elimination in figure gives the E-isomer and none of the Z-isomer.
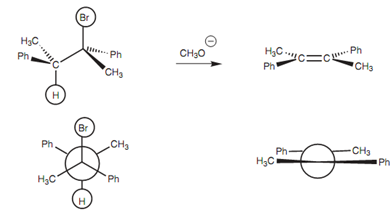
Figure: Stereochemistry of the E2 elimination reaction.