Oxidation:
The oxidation of alcohols is a very significant reaction in organic synthesis. Primary alcohols may be oxidized to aldehydes, but the reaction is tricky because there is the danger of over-oxidation to carboxylic acids. Along with volatile aldehydes, the aldehydes can be distilled from the reaction solution like they are formed.
Though, this is not possible for less volatile aldehydes. This problem can be conquers by using a mild oxidizing agent known as pyridinium chlorochromate (PCC). If a stronger oxidizing agent is employed in aqueous conditions (for example CrO3 in aqueous sulfuric acid), primary alcohols are oxidized to carboxylic acids (Fig. 12b), whereas secondary alcohols are oxidized to ketones.
The achievement of the PCC oxidation in stopping at the aldehyde stage is solvent dependent. The reaction is performed in methylene chloride, while oxidation with CrO3 is performed in aqueous acid.
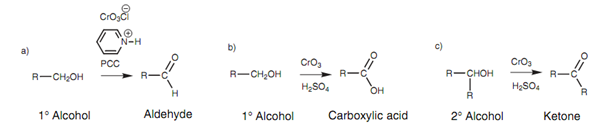
Figure: Oxidations of alcohols.