Mechanisms:
Definition
An understanding of nucleophilic and electrophilic centers permits a prediction of where reactions might take place but not what sort of reaction will happen. To understand and predict the outcome of reactions, it is essential to understand what goes on at the electronic level. This process is termed as a mechanism.
The mechanism is 'story' of how a reaction occurs. It describes how molecules react jointly to provide the last product. The mechanism tells us how bonds are created and how bonds are broken and what the order they carry is. It describes what is occurring to the valence electrons in the molecule because it is the movement of these electrons that result in a reaction. Take as a simple instance the reaction among a hydroxide ion and a proton to form water. The hydroxide ion is a nucleophile, whereas the proton is an electrophile. A reaction occurs among the nucleophilic center (the oxygen) and the electrophilic center (the hydrogen) and water is created. A new bond has been formed among the oxygen of the hydroxide ion and the proton. The mechanism looks at what takes place to the electrons.
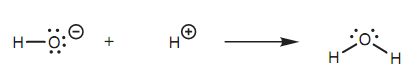
Figure: Reaction of a hydroxide ion and a proton to form water.