Hydroboration of 2-methylpropene:
With unsymmetrical alkenes, the least substituted alcohol is acquired (anti- Markovnikov) and thus the organoborane reaction is complementary to the electrophilic addition reaction with aqueous acid. Steric factors come out to play a role in controlling this preference with the boron atom preferring to approach the least sterically hindered site. Electronic factors as well play a role as explained in the mechanism below.
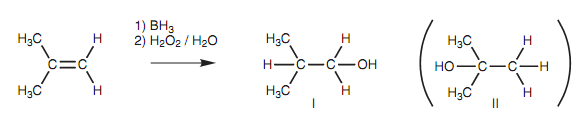
Figure: Hydroboration of 2-methylpropene to give a primary alcohol (I). The tertiary alcohol (II) is not obtained