Theory of Raman Spectroscopy:
An electromagnetic radiation when passed through a transparent medium interacts with the particles (e.g., molecules, atoms, or ions) constituting it. If the dimensions of the particles are equal to or smaller than that of its wavelength then it undergoes scattering. It has been observed that most of the scattered radiation has exactly the same wavelength as that of the incident radiation. Such a scattering is referred to as Rayleigh scattering. Therefore, an extremely small fraction (to the tune of about 1 in 107) of the scattered radiation is found to have a wavelength different from that of the incident radiation. This is called Raman scattering and the existence of Raman scattering is called Raman effect. Figure gives the spectra of the scattered radiation obtained when a sample of CCl4 was interacted with a laser beam having a wavelength of 488.0 nm.
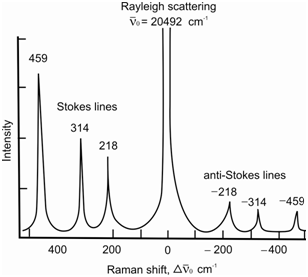
Figure: Raman spectra for CCl4 using 488 nm laser source
You might remember that the intense peak in the centre of the spectrum has the same wavelength (or wavenumber) as that of the incident radiation. This is the Rayleigh peak and other signals on either side of the Rayleigh peak are the Raman lines. The lines to the left of Rayleigh peak and having lower value of wavenumber are known as Stokes lines while the ones to the right and having higher value of wavenumber are called anti-Stokes lines. Stokes lines are at lower energy although the anti-Stokes lines are at energy greater than the Rayleigh peak.