Radiometric titration curve:
Conversely, NaCl may be
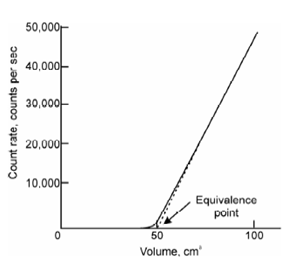
Figure: Radiometric titration curve using radiometric titrant
labeled with 36Cl (t1/2 = 3 × 105 γ) and its activity in solution may be monitored. In that case, however, plot shape will be reversed. In other words, activity will show decreasing trend with the addition of each drop of AgNO3 causing precipitation of Ag36Cl. At the end point, however, activity will become constant. End point can be determined from the point of inflexion. It may be noted that 36Cl is not a suitable radiotracer because of its long half-life.
As there is no method for titrating phosphate with a normal indicator, it may be conveniently determined by titrating with barium nitrate solution. For this purpose Na3PO4 labelled with 32P (t1/2 = 14.3d) is used and a GM counter may be employed for counting the activity. Instead of cumbersome apparatus, a number of centrifuge tubes may be taken. A graph may be plotted for a graph of counting rate on y-axis against volume of barium nitrate solution added as the x-axis, from the graph, concentration of sodium phosphate is calculated.