Procedure:
Typical experimental arrangement of radiometric titration consists of a titration vessel containing filter disc for phase separation as shown in Figure along with scintillation detector and lead shielding. A syringe is used to move supernatant solution into and out of the counting chamber which may be a scintillation detector.
A typical example of radiometric titration is for the determination of halides using 110Ag (t1/2 = 252 d) as tracer where corresponding silver halide is precipitated. In order to understand this method you may consider the titration of 10 mL of 1mM NaCl solution containing Cl- with 1 mM solution of 110AgNO3 to follow the reaction;
110AgNO3 + NaCl- → 110AgCl (ppt) + NaNO3
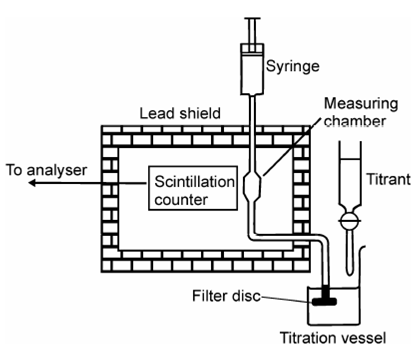
Figure: Illustration of experimental set-up for radiometric titration
where Ksp = 1.82 ×10-10 for AgCl. Activity of the supernatant solution is monitored after equilibrium is attained following the addition of titrant. Initially solution containing NaCl has no activity. With the addition of increasing amount of 110AgNO3, supernatant will have very little or insignificant activity because all the activity would have gone to the precipitate of 110AgCl. After the end point, however, activity of 110AgNO3 will come into the solution and keep increasing with every additional drop. Titration data are plotted as shown in Figure from where equivalence point is the intercept of the two straight lines of the curve.