Methodology:
Let us consider an analyte A with a binder B which can bind with A to form a complex A-B. A conventional methodology to determine analyte concentration is to add excess amount of reagent to form product which may give a direct measure of the analyte. For example, to determine the amount of Ag in a solution, excess amount of HCl is added to form the precipitate of AgCl which is weighed and thus amount of silver present in unknown sample is calculated. An alternative method could be to use radiotlabelled analyte and determine the activity of the product as in IDA. However, RIA differs in that instead of excess amount of reagent; only a limited or a little lesser amount of reagent is added as in substoichiometric isotope dilution analysis. Thus, only a fraction of analyte will get a chance to bind. In IDA, analyte is antigen (Ag) which is radiolabelled and the binder reagent is antibody (Ab).Determination of unknown concentration of antigen is based on the fact that the inactive and radiolabelled antigen competes physicochemically for the binding sites on the antibodies as illustrated in Figure. With increasing amount of analyte, this fraction or its activity decreases.In case of RIA, both inactive antigen (Ag) and radiolabellede antigen (Ag*) are taken in limited amount and compete with a fixed (but lesser than required for stoichiometric reaction) amount of antibody (Ab). The concentration of inactive Ag is varied. In the absence of inactive Ag, all the Ab will be consumed to form Ag*-Ab and may be considered as initial activity. As inactive Ag is added, fraction of Ag*-Ab and hence its activity will decrease. Thus, the amount of radioactivity associated with the complex is inversely related to the concentration of Ag. By using known amounts of Ag as standard, a standard curve is set up as shown in Figure. This may be used.
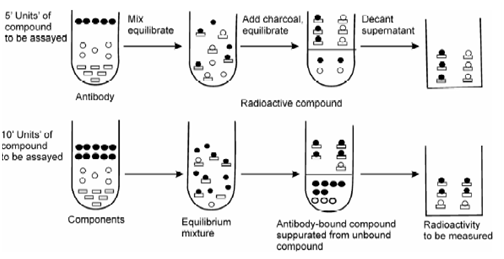
Figure: Diagram showing principle of radioimmunoassay
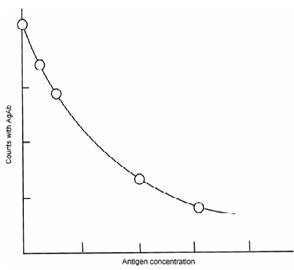
Figure: Standard curve for radioimmunoassay used for the determination of an antigen