Radioactive:
The ratio of neutrons to protons is frequent used as an indicator of the stability of a nucleus. If atomic numbers (Z) of all stable nuclei are plotted as a function of the number of neutrons (N) present within their nuclei, a plot shown in Figure is acquired. It displays in which all stable light elements up to Z = 20, need approximately equal number of neutrons and protons. While there are some exceptions such as 2D, 13C, 17O, 18O, 19F, 23Na, 27Al, 31P where this rule of Z = N is not applicable. For the heavier elements (beyond Z >20) considerably more number of neutrons are required. This deviation from N/Z = 1 occurs since protons being charged particles repel each other (Coulomb's law).
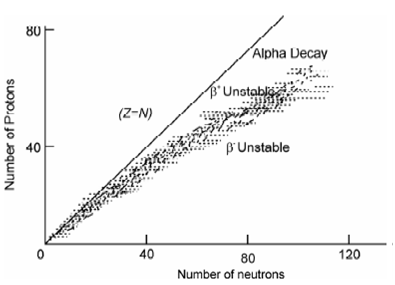
Figure: Plot of number of neutrons vs. number of protons in stable nuclides
Because nucleons are held together inside nucleus through strong and short range nuclear forces, additional forces (through adding number of neutrons) are required to compensate Coulomb repulsion forces. For stable nuclides N/Z varies in a huge range of 1 for 40Ca to 1.54 for 202Hg as evident from data given in Table 12.1. Though N/Z = 1 up to Z=20 but there are a few exceptions such as 7Li, 9Be,
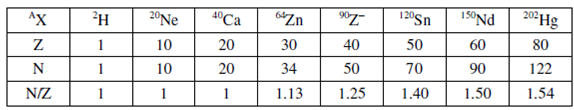
Table : Variation of N/Z ratio with increasing Z
11B, 13C, 15N, 17O, 18O, 19F, 23Na, 25Mg, 27Al, 29Si, 31P, 34S, 40Ar, 41K, 42Ca which all have N/Z >1. Also there are a few stable pair of nuclides along with odd Z and mass numbers A and A+2, e.g. 35Cl - 37Cl, 39K - 41K, 63Cu - 65Cu, 79Br - 81Br, -85Rb - 87Rb, 107Ag - 109Ag. Additional, there are several stable nuclides that occur in nature as monoisotopic e.g. 19F, 23Na, 27Al, 31P, 45Sc, 55Mn, 59Co, 75As, 127I, 197Au etc.