Spectra of phenanthrene:
Since the fluorescence emission takes place only after the excited molecule has relaxed to the vibrational ground state of the S1 state that is after having lost part of its energy, the energy of the emitted radiation is lower than which of the excitation radiation. This means that the wavelength of fluorescence emission would be greater than the excitation wavelength. In addition, since the energy of the triplet excited state of the molecule is lower than in which of the associated singlet state; the transitions to the ground state are associated along with the emission of light of still lower energy. As a consequence the phosphorescence occurs at longer wavelengths than fluorescence. Figure displays the excitation, fluorescence and phosphorescence spectra of phenanthrene
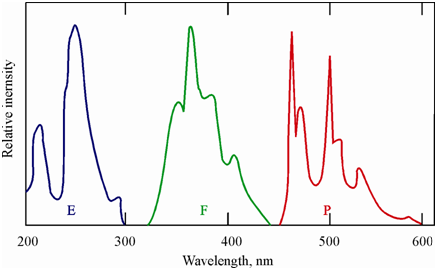
Figure: The excitation (E), fluorescence (F) and phosphorescence (P) spectra of phenanthrene
The excited molecules while reach a triplet state, spend associative long time in that state. Because they are at the triplet state for a long time, they could lose their energy through means other than through emission of photon i.e., they are extremely susceptible to collisions along with the solvent molecules. Thus, phosphorescence in solution at room temperature is a rare phenomenon. Therefore, the phosphorescence can be observed through decreasing the temperature of the sample whereby decreasing the collisions along with the solvent molecules. Further, the quenching of the triplet excited state through oxygen is also responsible in avoiding phosphorescence, and thus the solution of the analyte requires to be thoroughly degassed before determination of its phosphorescence.