Radiation Effects-II
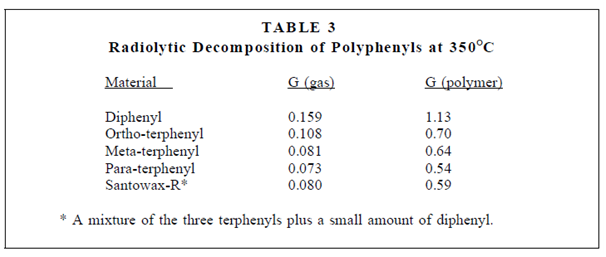
The stability of organic (and other covalent) compounds to radiation is often expressed through means of the "G" value, that is equivalent to the number of molecules decomposed, or of product established, per 100 eV of energy dissipated in the material. As an instance of the use of G values, a data in Table are for a number of polyphenyls exposed to the radiation in a thermal reactor.
The table displays the number of gas molecules produced, G (gas), and the number of polyphenyl molecules, G (polymer), used to generates higher polymers per 100 eV of energy deposited in the material. Remember that this adds up to around 1000 atoms of gas and 10,000 atoms forming higher polymers per every 1 MeV particle. It is also of interest to note in which the terphenyls are even more resistant to radiation than diphenyl and because they have a higher boiling point, a combination of terphenyls along with an associatively low melting temperature was selected as the moderator- coolant in organic-moderated reactors.
An effect same to that describe above occurs in water molecules in which are decomposed through radiation into hydrogen and oxygen in a reactor. Control of oxygen generates through this procedure is an important category of reactor chemistry.