Validation of Beer and Lambert's law:
Now let us rewrite the expression for the Beer and Lambert's law as
log Po / P = A= abc or εbc
According to this expression, if we know the values of a (or ε) and b then we could determine the concentration straight from the absorbance value. Therefore, commonly the value of ε is not known accurately for the species being determined under the conditions of the determination. Quite frequent even the validity of the Beer and Lambert's law expression is questionable. Thus in most of the methods, a calibration curve are acquired through measuring the absorbance values for a series of standard solutions of the analyte being determined at a fixed wavelength. These solutions should be under same solution conditions and in the range of the concentration of the analyte. For the law to be valid, the plot of absorbance, A versus the concentration, c for the standard solutions should be a straight line passing by origin.
Such a curve is called as calibration curve and is handy within determination of concentration of unknown solutions from a measurement of absorbance value. For instance, the unknown concentration of a solution of iron can be determined through using a calibration curve acquired through plotting the absorbance values of a series of standard solutions of iron measured at 562 nm depicted in Figure (a).
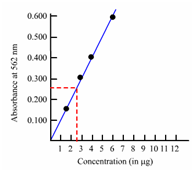
Figure: Calibration curve: a) Standard solution method