NMR spectrum of phenol:
Phenol is another typical case because of the acidic nature of the -OH group unlike other hydroxyl compounds discussed above. The nature of spectrum is strongly concentration dependent. At ordinary concentration, a strong sharp signal is observed in the range 6.0 to 7.7 ppm. However, at low concentration or for dilute solutions, signal is shifted upfield in the range 4 to 5 ppm. Variation of phenolic hydroxyl signal with concentration is shown in Figure.
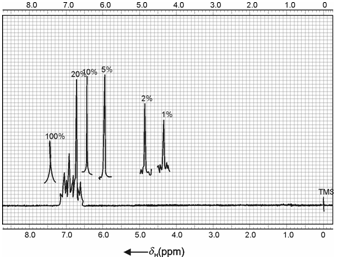
Figure: NMR spectrum of phenol, 20% (w/v) in CCl4. Also shown is variation of hydroxyl absorptions at various concentrations for pure, 10%, 5%, 2% and 1% solutions
The hydroxyl signal for pure phenol is observed at 7.45 ppm which at 20% (w/v) in carbon tetrachloride is shifted to upfield at 6.75 ppm. The small peaks observed by the side of -OH signal may be attributed to aromatic benzene protons. On further dilutions at 10, 5, 2 and 1% concentrations, OH signal is continuously shifted to upfield at 6.45, 5.95, 4.88 and 4.37 ppm respectively. This shift can be attributed to loss of intermolecular hydrogen bonding on dilution.