NMR spectrum of benzyl alcohol:
You may note that as the temperature is lowered from 31oC to 6oC and then to 1oC no splitting occurs in any of the two lines though both lines get broadened; the broadening in OH signal being more prominent. On further lowering the temperature down to -4oC broadening becomes more significant suggesting some kind of interaction which, however, becomes clear at - 6oC. At -14oC, methyl group signal shows a doublet (J = 5.2 Hz) due to spin-spin interaction with OH proton. At the same time OH signal also shows a doublet but with some structure on both sides. On further lowering of temperature to -40oC, a clear quartet (J = 5.2 Hz) is observed for the hydroxyl proton.
This kind of observation can be explained in terms of chemical exchange. The observed spectra indicate that at room temperature rate of chemical exchange is very fast giving rise to two sharp singlets. However, at - 40oC, rate of chemical exchange is very slow giving rise to multiplets arising out of spin-spin coupling.
Benzyl alcohol: In order to understand the effect of benzene ring in alcohols, let us take the example of benzyl alcohol. The spectrum of benzyl alcohol recorded at 60 MHz is shown in Figure. In the two cases discussed above you have seen that OH signal is observed at down fields due to O atom being electronegative. However, in the case of C6H5CH2OH, the OH signal is observed quite upfield (δ = 2.5 ppm) and the benzyl group downfield (δ = 7.2 ppm) with methylene group (-CH2-) in between the two (δ = 4.6 ppm). You know that the downfield shift of benzyl group protons is due to the anisotropic effect of the benzene ring. In none of these cases, however, any multiplicity is observed.
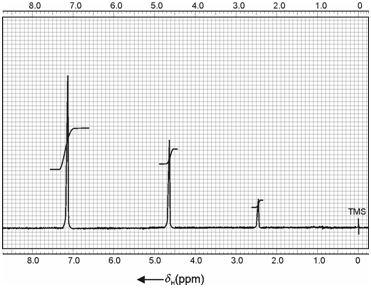
Figure: NMR spectrum of benzyl alcohol (C6H5CH2OH) in CCl4 at low resolution