NMR spectra of methanol:
The rate of proton transfer in pure ethanol is relatively slow and the proton is available on the oxygen atom and can interact with the methylene protons giving a triplet. However, in presence of acidic or basic impurities ordinarily present in the sample the rate of exchange is markedly increased and the proton does not reside on the oxygen atom for sufficiently long to interact with methylene group and only a sharp singlet is observed as shown in Figure.
Methanol is a very simple case as far as NMR spectrum is concerned. It is a good example to demonstrate chemical exchange at high temperatures. At room temperature, NMR spectrum of methanol (CH3-OH) as expected, exhibits two lines. A sharp signal at high field is due to methyl group (CH3) and a low intensity downfield signal is due to alcoholic proton (OH). However, on lowering the temperature of measurement it shows interesting changes that are attributed to the exchange phenomenon. The NMR spectra of CH3OH at varying temperatures of 31, 6, 1, 4, 6, 14, and 40oC are shown in Figure.
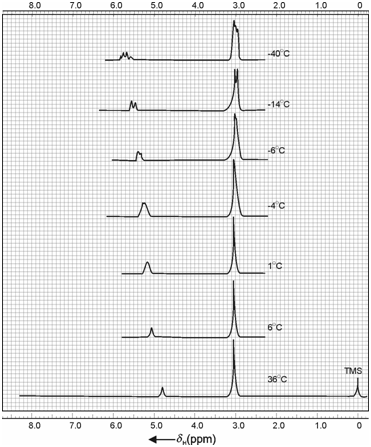
Figure: NMR spectra of methanol at varying temperatures