Spin-spin coupling:
Till now, we have looked at spectra in which each signal is a single peak (singlet). Though, in many spectra, the signal for a specific proton is made up of two or more peaks. This is because of an effect termed as spin-spin coupling that is generally seen when non-equivalent protons are on neighboring carbon atoms. An instance of this can be seen in the nmr spectrum of the alkene displayed in figure.
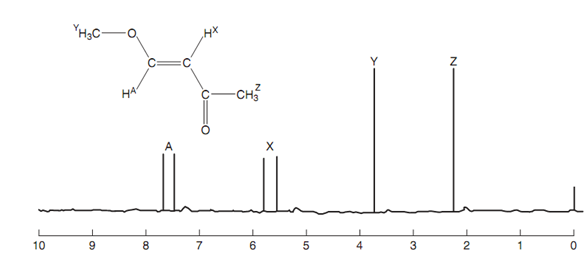
Figure: NMR spectrum of a disubstituted alkene.
There are 4 non-equivalent protons present in this molecule and thus we would expect four signals. The spectrum does certainly depict four signals even though there are six peaks present. The signals at 2.2 ppm and 4.7 ppm are because of the two methyl groups and are both singlets. Though, the signals for the two alkene protons are both split into doublets. So, the two peaks between 5 and 6 ppm constitute one signal because of HX. The chemical shift of this signal is the midpoint between the two peaks that is at 5.65 ppm. Likewise the two peaks between 7 and 8 ppm constitute one signal because of HA, with the chemical shift being the midpoint among the two peaks at 7.6 ppm.