Secondary magnetic due to electrons:
The above figure shows an isolated proton spinning and precessing in an applied magnetic fields field. Thus far we have only considered the nucleus of the hydrogen atom; however we know that electrons must be available. Let us refer the effect of one electron orbiting the nucleus as shown in figure.
As the electron is a spinning, charged body, electron sets up a secondary magnetic field of its own (Be). The secondary magnetic field (Be) opposes the external magnetic field (Bo). So the nucleus is shielded from the external magnetic field. Here this means the actual magnetic field experienced through the nucleus is reduced (Bo-Be). As the nucleus experiences a reduced magnetic field, the precessional / Larmor frequency is reduced. In turn this means that less energy is needed to make that nucleus resonate and provide a signal. The larger the electron density round a proton, the larger the shielding and thus the position of a signal in an nmr spectrum can be an indication of electron density in dissimilar parts of a molecule.
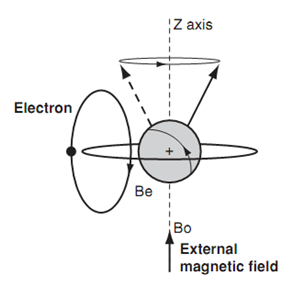
Figure: Secondary magnetic field from an electron.