Larmor frequency and energy transitions:
To acquire a spectrum we need to get transitions among the two energy levels. This can be acquired by firing in a burst of energy in the kind of electro- magnetic radiation. The influence of this energy is to excite the nucleus and to reason it to '?ip' such that it is now against the magnetic field. This orientation is much less stable as compared to the original orientation and thus the nucleus has absorbed energy. It is found that the energy needed to do this has similar frequency like the Larmor frequency. If the electromagnetic radiation is now stopped, the nucleus relaxes back to the much more stable orientation. The result of it is that the energy is emitted. Such type of energy can be detected and calculated leading to a signal in an nmr spectrum. The energy difference among the two orientations is very small and is in the radiofrequency region of the electromagnetic spectrum. The energy variation is proportional to the Larmor frequency that is proportional to the strength of the external applied magnetic field. In a magnetic field of 14 100G, the energy variation is 60 × 106 Hz or 60 MHz. In a magnetic field of 23 500G the energy difference is 100 MHz. NMR spectrometers act at a particular magnetic field and thus a particular electromagnetic radiation is needed for resonance. The convention is to recognize the spectrometers and their spectra through the strength of the electromagnetic radiation employed (that is 60 MHz or 100 MHz).
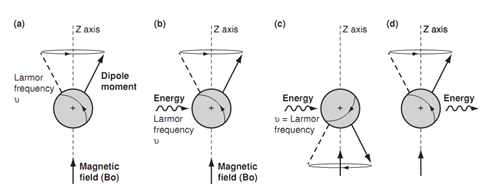
Figure: Larmor frequency and energy transitions.