Inductive effects on chemical shift:
TMS is called an internal reference because it is dissolved in the deuterated solvent employed to dissolve the sample. There is a good cause why TMS is employed like an internal reference. Silicon has a trend to 'repel' the electrons in the silicon- carbon bonds such that they are pushed in the direction of the methyl groups - an inductive effect. Here this means that the protons in these methyl groups practice a high electron density which shields the nuclei and results in a low chemical shift, lower in fact than the huge majority of nmr signals observed in organic molecules.
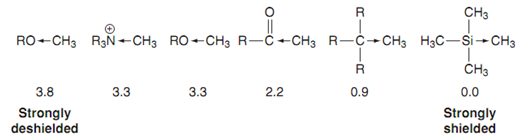
Figure: The inductive effect and chemical shift.
Inductive effects have a significant effect on chemical shift. We can observe this with a series of methyl groups. Going from right to left we have TMS that sets the scale at 0 ppm. After that we have the methyl group of a saturated hydrocarbon. The alkyl groups of a hydrocarbon as well 'push electrons' away from them and therefore increase electron density round a neighboring methyl group. Though this inductive effect is not as powerful as the one caused via a silicon atom and thus the methyl signals in hydrocarbons generally take place about 0.9 ppm.