Proteins of the endoplasmic reticulum:
The RER contains several proteins which have the role of assisting nascent proteins to fold rightly into their native conformation. Some of these are known as chaperones. RER-resident proteins are made on the RER and either pass into the lumen (as do secretory proteins) or are anchored in the membrane as for Type I integral membrane proteins. Moreover, these proteins contain a retention signal at the C terminus which is recognized through specific receptor proteins which retain these proteins in the RER, avoiding them from moving along the secretory pathway to the Golgi. In case of the soluble proteins in the lumen of the RER the retention signal is Lys-Asp-Glu-Leu or KDEL using the one-letter amino acid code at the C terminus. In case of the Type I integral membrane proteins in the RER membrane the retention signal is KKXX (Lys-Lys-Xaa-Xaa) in the cytosolic C terminus.
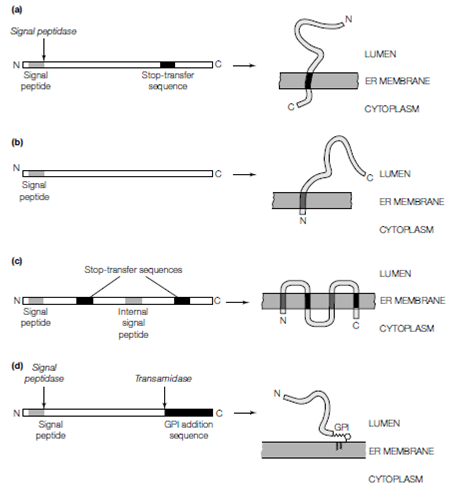
Figure: Insertion of integral membrane proteins into the RER membrane during synthesis. (a) Type I integral membrane protein with a cleavable N-terminal signal sequence and a stop-transfer sequence; (b) Type II integral membrane protein with an uncleaved N-terminal signal sequence; (c) Type III integral membrane protein with multiple signal and stop- transfer sequences; (d) glycosyl-phosphatidylinositol (GPI) anchored membrane protein with a cleavable N-terminal signal sequence and a C-terminal GPI anchor addition sequence.