Secondary structure
Introduction: In a protein Secondary structure refers to the regular folding of regions of the polypeptide chain. Two most common types of secondary structure are the the β-pleated & α-helix sheet. The α-helix is a cylindrical, rod-like helical arrangement of the amino acids which is maintained in the polypeptide chain by hydrogen bonds parallel to the helix axis. In a β-pleated sheet, hydrogen bonds composed between adjacent sections of polypeptides that are either running in the similar direction (parallel β-pleated sheet) or in the opposite direction (antiparallel β- pleated sheet).The β-Turns reverse the direction of the polypeptide chain and are frequently found connecting the ends of antiparallel β-pleated sheets.
In a protein the secondary level of structure is the regular folding of regions of the polypeptide chain. Two most common types of protein fold are the α- helix and the β-pleated sheet. In the rod-like α-helix, the amino acids set up themselves in a regular helical conformation .The carbonyl oxygen of each peptide bond on the amino group is hydrogen bonded to the hydrogen of the fourth amino acid away , having the hydrogen bonds running closely parallel to the axis of the helix. There are 3.6 amino acids per turn of the helix covering a distance of 0.54 nm, in a α-helix and each amino acid residue represents an advance of 0.15 nm along the axis of the helix. The amino acids’ side-chains are all positioned along the outside of the cylindrical helix .Certain amino acids are more frequently found in α-helices than others. In specific, Pro is hardly ever found in α-helical regions as due to the lack of a hydrogen atom on its nitrogen atom it cannot form the accurate pattern of hydrogen bonds., Pro is frequently found at the end of an α-helix, For this reason where it change the direction of the polypeptide chain and terminate the helix. Different proteins have a different amount of the polypeptide chain folded up into α-helices. For instance, the single polypeptide chain of myoglobin has 8 α-helices.
Hydrogen bonds form between the peptide bonds In the β -pleated sheet either in different polypeptide chains or in different sections of the similar polypeptide chain . The planarity of the peptide bond forces the polypeptide to be pleated having the side-chains of the amino acids protruding above and below the sheet. Neighbouring polypeptide chains in β-pleated
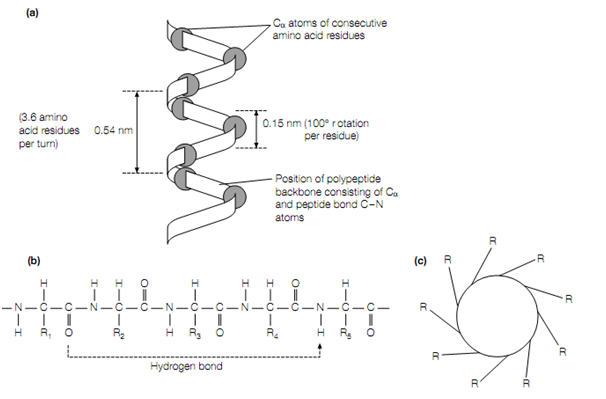
Figure:The folding of the polypeptide chain into an α-helix. (a) Model of an α-helix having only the Cα atoms along the backbone shown; (b) n is hydrogen bonded to the NH group on residue (n + 4) in the α-helix the CO group of residue; (c) cross-sectional view of an α-helix showing the positions of the side-chains (R groups) of the amino acids on the outside of the helix.
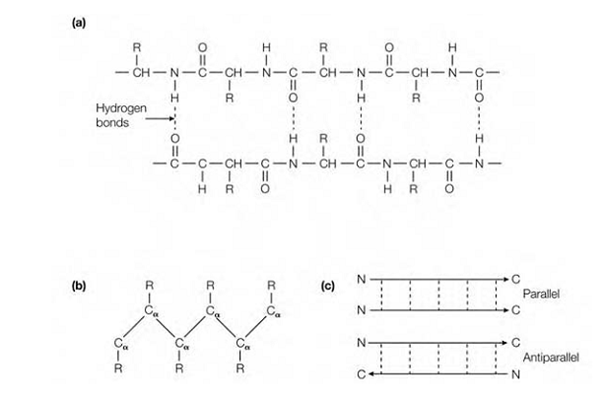
Figure:The folding of the polypeptide chain in a β-pleated sheet. (a) Hydrogen bonding between 2 sections of a polypeptide chain composing a β-pleated sheet; (b) a side-view of one of the polypeptide chains in a β-pleated sheet indicating the side-chains (R groups) connect to the Cα atoms protruding above and below the sheet; (c) because the polypeptide chain has polarity, either parallel or antiparallel β-pleated sheets can form.
sheets may be either parallel or antiparallel based on whether they run in the similar direction or in opposite directions, respectively . The polypep- tide chain within a β-pleated sheet is completely extended, such as there is a distance of 0.35 nm from one Cα atom to the next. Always Β-Pleated sheets are slightly curved and, if many polypeptides are involved, the sheet can bind to form a β-barrel. Multiple β-pleated sheets provide rigidity and strength in various structural proteins, like silk ?broin, which consists almost wholly of stacks of antiparallel β-pleated sheets.
For the purpose of fold tightly into the compact shape of a globular protein, the polypeptide chain frequently reverses direction, making a hairpin or β -turn. The carbonyl oxygen of one amino acid is hydrogen bonded to the hydrogen on the amino group of the fourth amino acid along In these β-turns .β-Turns
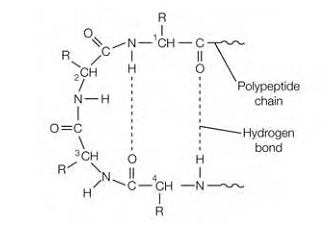
Figure:In a β-turn ,the folding of the polypeptide chain.
are frequently found connecting the ends of antiparallel β-pleated sheets. Regions of the polypeptide chain that are not in a regular secondary structure are called to have a coil or loop conformation.