Peptide bond
Introduction: A protein is a linear sequence of amino acids linked by peptide bonds together. The peptide bond is a covalent bond between the α-carboxyl group of another and the α-amino group of one amino acid. The peptide bond has partial double bond character and is closely in the trans configuration always. Backbone conformation of a polypeptide is defined by the rotation angles around the Cα–N bond (phi, φ) and Cα–C bond (psi, ψ) of each of its amino acid residues. The sterically permitted values of ψ and φ are visualized in a Ramachandran plot. When by a peptide bond 2 amino acids are joined they form a dipeptide. In Addition of further amino acids results in long chains called and polypeptides and oligopeptides .
Proteins are linear sequences of amino acids which are linked together by peptide bonds. The peptide bond is a type of chemical, covalent bond formed between the α-amino group of one amino acid and the α-carboxyl group of another amino acid. Once two amino acids are joined together using a peptide bond to form a dipeptide, there is yet a free amino group at one end and a free carboxyl group at the other, each of which may in turn be linked to further amino acids. Therefore, to form oligopeptides (up to 25 amino acid residues) and polypeptides (> 25amino acid residues) long, unbranched chains of amino acids can be linked by peptide bonds together. Note that the polypeptide has a free α-amino group still and a free α-carboxyl group. The Convention has it that peptide chains are written down having the free α-amino group on the left, the free α-carboxyl group on the right and a hyphen between the amino acids to show the peptide bonds.
Therefore, the tripeptide +H3 N-serine–leucine–phenylalanine-COO– should be written easily as Ser-Leu-Phe or S-L-F.
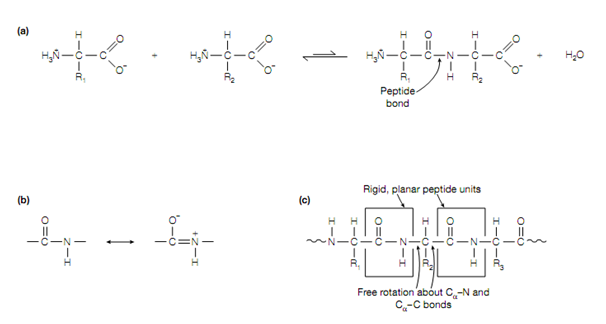
Figure: (a) Formation of a peptide bond, (b) resonance structures of the peptide bond, (c) peptide units within a polypeptide.
between the carbon and nitrogen the peptide bond exhibits partial double- bond character due to the nearness of the carbonyl carbon–oxygen double-bond permitting the resonance structures in given fig to exist. The C–N bond length is also shorter than usual C–N single bonds because of this. The peptide unit which is made up of the CO–NH atoms is therefore relatively planar and rigid , however, free rotation may take place about the Cα–N and Cα–C bonds (the bonds either side of the peptide bond), allowing adjacent peptide units to be at different angles . Always the hydrogen of the amino group is nearly on the opposite side (trans) of the double bond to the oxygen of the carbonyl group, rather than on the similar side (cis).
The backbone of a protein is a connection sequence of rigid planar peptide groups. Backbone conformation of a polypeptide is defined by torsion angles or rotation angles about the Cα–N bond (phi, φ) and Cα–C bond (psi, ψ) of each of its amino acid residues. When the polypeptide chain is in its planar, fully extended (all-trans) conformation the φ and ψ angles are both specified as 180 , and amplify for a clockwise rotation when viewed from Cα .The conformational range of the torsion angles, ψ and φ are restricted by steric hindrance in a polypeptide backbone . The sterically permitted values of ψ and φ can be finding out by calculating the distances between the atoms of a tripeptide at all values of ψ and φ for the central peptide unit. These values are visualized, or else known as a conformation map or Ramachandran plot In a steric contour diagram .From given figure it may be seen that most areas of the Ramachandran plot (most combinations of ψ and φ) are conformation ally inaccessible to a polypeptide chain. Three small regions of the conformation map are physically accessible to a polypeptide chain Only, and within these regions are the φ–ψ values that generate the right-handed α-helix, the parallel and antiparallel β-pleated sheets and the collagen helix .
To form a particular shape the polypeptide chain folds up (conformation) in the protein.In the structure this conformation is the three-dimensional arrangement of atoms and is determined by the amino acid sequence. There are 4 levels of structure in proteins: primary, secondary, tertiary and, sometime quaternary (but not always).
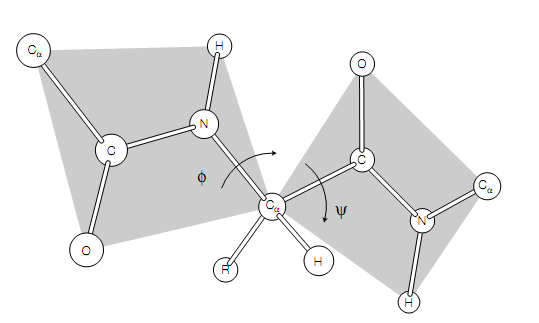
Figure:A segment of a polypeptide chain indicating the torsion angles about the Cα–N bond (φ) and Cα–C bond (ψ).
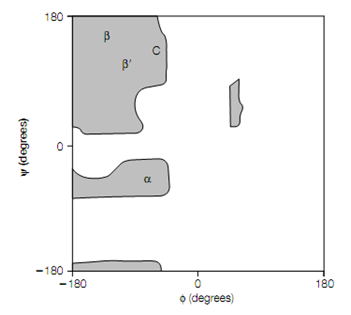
Figure:Ramachandran plot indicating the allowed angles for poly-L-alanine (grey regions). α, φ–ψ values that generate the right-handed α-helix; β, antiparallel β-pleated sheet; β , parallel β-pleated sheet; C, collagen helix.