Ion exchange chromatography
Proteins are separated on the basis of their whole (net) charge in ion exchange chromatography if a protein has a net negative charge at pH 7, it will connect to a column containing positively-charged beads, while a protein with no charge or a net positive charge will not connect. The negatively-charged proteins bound to such a column can then be eluted through washing the column with an rising gradient (rising concentration) of a solution of sodium chloride (Na+ Cl– ions) at the appropriate pH. For the positively-charged groups on the column the Cl– ions compete with the protein. Proteins having a low density of negative charge elute ?rst, followed through those with a higher density of negative charge. Columns containing positively-charged diethylaminoethyl (DEAE) collection (like as DEAE-cellulose or DEAE-Sephadex) are used for separation of negatively-charged proteins (anionic proteins).
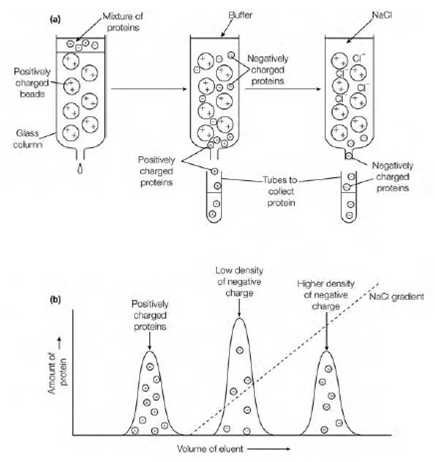
Figure: Ion exchange chromatography. (a) Schematic illustration of ion exchange chromatography; (b) elution diagram indicating the separation of a protein of net positive charge that does not bind to the positively-charged beads and passes straight through the column, and of two proteins with different net negative charges that bind to the positively-charged beads and are eluted on increasing the concentration of NaCl applied to the column. The protein with the lower density of negative charge elutes earlier than the protein with the higher density of negative charge.
This is known as anion exchange chromatography. Columns containing negatively-charged CM (carboxymethyl) groups (like as CM-cellulose or CM-Sephadex) are used for the separation of positively-charged proteins (cationic proteins). This is known as cation exchange chromatography. As an instead to elution with a gradient of NaCl proteins can be eluted from anion replaced columns through decreasing the pH of the buffer and from cation exchange columns through increasing the pH of the buffer, therefore altering the ionization state of the amino acid side-chains and therefore the net charge on the protein.