Pressure-Temperature (P-T) Diagram
A P-T diagram is the most general way to illustrate the phases of a substance. Figure below is the P-T diagram for pure water. A P-T diagram can be built for any pure substance. The line which divides the solid and vapor phases is termed as the sublimation line. The line which divides the solid and liquid phases is termed as the fusion line. The line which divides the liquid and vapor phases is termed as the vaporization line. The point where three lines met is termed as the triple point. The triple point is just a point at which all the three phases can exist in symmetry. The point where the vaporization line finishes is termed as the critical point. At temperatures and pressures more than those at the critical point, no substance can exist as a liquid any issue how huge a pressure is applied upon it.
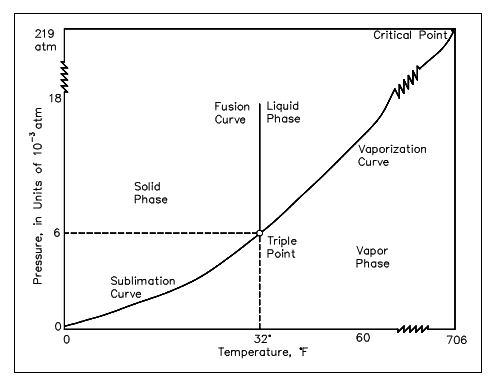
Figure: Diagram for Water