Molecular Weights and Gas Constants for Dry Air and Water Vapour:
From the respective mole fractions & molecular masses of component gases, the molecular mass of the dry air portion might be computed.
Since a portion of volume represents the mole fraction. By using the values for mole fraction from given Table: We contain molecular Mass of dry air
Ma = ∑ My ... 6.9
28.02 (0.7803) + 32 (0.2099) + 39.91 (0.0094) + 44(0.0003) + 2.02 (0.0001)
= 28.966
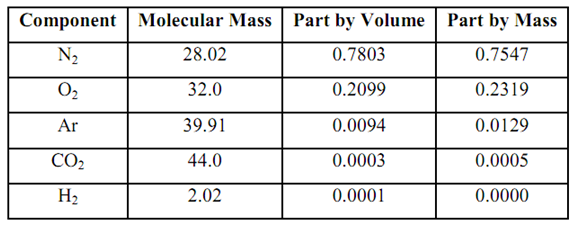
Composition of Dry Part in Atmospheric Air
By knowing that the value of the universal gas constant is 8.3143 kJ/kg mole K, the gas constants used for the two parts of moist air are as following:
Dry air
Ma= 28.966
R = 8.3143/ 28.966
= 0.2871 kJ/kg. K
Water vapour
Mv= 18.016
R = 8.3143/18.016
=0.461 kJ/kg. K
here subscript v refers to water vapour.