Mole Fractions of Component Gases:
The ratio of partial pressure to net pressure, & volume fraction are equivalent to the mole fraction of the gas.
Consider
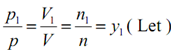 ...6.4
...6.4
Molecular Mass of Mixture
As m = m1 + m2, and m = Mn, m1 = M1 n1, m2 = M2 n2, we obtain
Mn = M1 n1 + M2 n2
Therefore
M = y1M1 + y2 M2 or M = ∑i yi Mi ...6.5
here M refers the molecular mass of the mixture and n1 = m1/M1, n2= m2/M2, etc. likewise, for the mixture, n = m/M.
Gibbs' Theorem:
Gibbs' Theorem additionally enunciates that the internal energy of a mixture is equivalent to the total of internal energies of the specific components, taken each at the temperature & volume of the mixture. Therefore, we have for the internal energy of the mixture.
mu = m1u1 + m2u2 ... 6.6
This can also be illustrate that the enthalpy & specific heat of the mixture can, likewise, be written as following
mh = m1h1 + m2h2 ... 6.7
mc = m1c1 + m2c2 ... 6.8